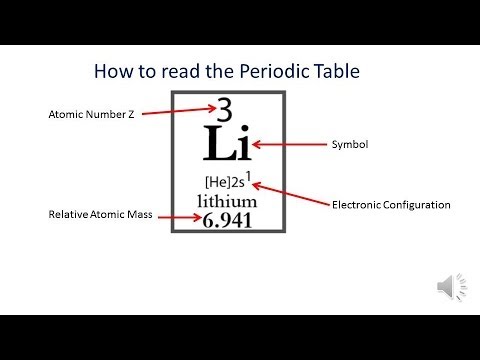
Magnesium is a chemical element with atomic number 12 which means there are 12 protons and 12 electrons in the atomic structure. The chemical symbol for Magnesium is Mg. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. The nucleus is composed of protons and neutrons.
| 1 | Hydrogen | H |
| 2 | Helium | He |
| 3 | Lithium | Li |
| 4 | Beryllium | Be |
| 5 | Boron | B |
| 6 | Carbon | C |
| 7 | Nitrogen | N |
| 8 | Oxygen | O |
| 9 | Fluorine | F |
| 10 | Neon | Ne |
| 11 | Sodium | Na |
| 12 | Magnesium | Mg |
| 13 | Aluminum | Al |
| 14 | Silicon | Si |
| 15 | Phosphorus | P |
| 16 | Sulfur | S |
| 17 | Chlorine | Cl |
| 18 | Argon | Ar |
| 19 | Potassium | K |
| 20 | Calcium | Ca |
| 21 | Scandium | Sc |
| 22 | Titanium | Ti |
| 23 | Vanadium | V |
| 24 | Chromium | Cr |
| 25 | Manganese | Mn |
| 26 | Iron | Fe |
| 27 | Cobalt | Co |
| 28 | Nickel | Ni |
| 29 | Copper | Cu |
| 30 | Zinc | Zn |
- The number of occupied shells corresponds to the atom's atomic number. An electron configuration is unique to each element, much like its atomic spectrum. If the atom is electrically neutral, the number of electrons corresponds to the atom's atomic number.
- Holonyms ('atomic number 12' is a substance of.): carnallite (a white or reddish mineral consisting of hydrous chlorides of potassium and magnesium; used as a fertilizer and as a source of potassium and magnesium).
- Four elements P, Q, R and S have atomic numbers 12,13,14 and 15 respectively. Answer the following questions giving reasons: (i) What is the valency of Q? (ii) Classify these elements as metals and non-metals.
| 31 | Gallium | Ga |
| 32 | Germanium | Ge |
| 33 | Arsenic | As |
| 34 | Selenium | Se |
| 35 | Bromine | Br |
| 36 | Krypton | Kr |
| 37 | Rubidium | Rb |
| 38 | Strontium | Sr |
| 39 | Yttrium | Y |
| 40 | Zirconium | Zr |
| 41 | Niobium | Nb |
| 42 | Molybdenum | Mo |
| 43 | Technetium | Tc |
| 44 | Ruthenium | Ru |
| 45 | Rhodium | Rh |
| 46 | Palladium | Pd |
| 47 | Silver | Ag |
| 48 | Cadmium | Cd |
| 49 | Indium | In |
| 50 | Tin | Sn |
| 51 | Antimony | Sb |
| 52 | Tellurium | Te |
| 53 | Iodine | I |
| 54 | Xenon | Xe |
| 55 | Cesium | Cs |
| 56 | Barium | Ba |
| 57 | Lanthanum | La |
| 58 | Cerium | Ce |
| 59 | Praseodymium | Pr |
| 60 | Neodymium | Nd |
| 61 | Promethium | Pm |
| 62 | Samarium | Sm |
| 63 | Europium | Eu |
| 64 | Gadolinium | Gd |
| 65 | Terbium | Tb |
| 66 | Dysprosium | Dy |
| 67 | Holmium | Ho |
| 68 | Erbium | Er |
| 69 | Thulium | Tm |
| 70 | Ytterbium | Yb |
| 71 | Lutetium | Lu |
| 72 | Hafnium | Hf |
| 73 | Tantalum | Ta |
| 74 | Tungsten | W |
| 75 | Rhenium | Re |
| 76 | Osmium | Os |
| 77 | Iridium | Ir |
| 78 | Platinum | Pt |
| 79 | Gold | Au |
| 80 | Mercury | Hg |
| 81 | Thallium | Tl |
| 82 | Lead | Pb |
| 83 | Bismuth | Bi |
| 84 | Polonium | Po |
| 85 | Astatine | At |
| 86 | Radon | Rn |
| 87 | Francium | Fr |
| 88 | Radium | Ra |
| 89 | Actinium | Ac |
| 90 | Thorium | Th |
| 91 | Protactinium | Pa |
| 92 | Uranium | U |
| 93 | Neptunium | Np |
| 94 | Plutonium | Pu |
| 95 | Americium | Am |
| 96 | Curium | Cm |
| 97 | Berkelium | Bk |
| 98 | Californium | Cf |
| 99 | Einsteinium | Es |
| 100 | Fermium | Fm |
| 101 | Mendelevium | Md |
| 102 | Nobelium | No |
| 103 | Lawrencium | Lr |
| 104 | Rutherfordium | Rf |
| 105 | Dubnium | Db |
| 106 | Seaborgium | Sg |
| 107 | Bohrium | Bh |
| 108 | Hassium | Hs |
| 109 | Meitnerium | Mt |
| 110 | Darmstadtium | Ds |
| 111 | Roentgenium | Rg |
| 112 | Copernicium | Cn |
| 113 | Nihonium | Nh |
| 114 | Flerovium | Fl |
| 115 | Moscovium | Mc |
| 116 | Livermorium | Lv |
| 117 | Tennessine | Ts |
| 118 | Oganesson | Og |
Download a printable version of the Periodic Table of Elements in PDF format:
- Color: Basic / Advanced
- Black and White: Basic / Advanced
The following on-line games based on the Periodic Table of Elements are available:
- Element Flash Cards
- Element Hangman

- Element Matching
- Element Math
- Element Crossword Puzzles

- Element Concentration
- Element Balancing
- Element Word Scramble
The following paper-based activities are available:
- Element BINGO
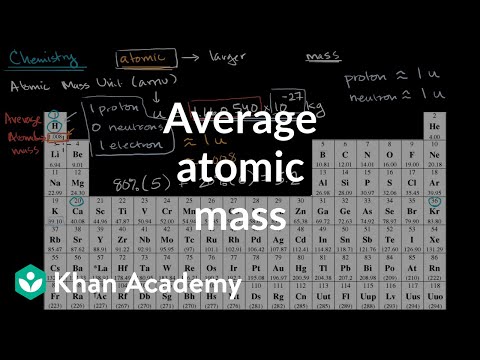
Carbon 12 Atomic Mass
- Element Word Search
In addition to the information contained within the Periodic Table of Elements, the following articles may be helpful if you are writing a report about an element or if you are making a model of an atom:
- How to calculate the number of protons, neutrons and electrons in an atom of an element
- How to make a model of an atom
- How to draw an atom (video)
- How to read an electron configuration chart
Atomic Number 120
- A list of who discovered each element
Atomic Number 12 Mass Number 24
The information on this site has been compiled from a number of sources.
Atomic Number 127
For questions about this page, please contact Steve Gagnon.
